
TECHNOLOGY
- SynThese™
Genome Engineering For Humanity - Next-generation Transgenic Mouse Platform for Fully Human Antibody Drug Discovery
SynThese™ Antibody Discovery Platform
Development of fully human antibody drug candidates against the specific antigen and the assessment of drug efficacy and safety.
Establishment of fully potentiated mature mAb library that is naturally selected from transgenic mouse platform.
* Antibody discovery using transgenic mouse platform is recognized as a top-notch technology, displaying a technical superiority over conventional Phage display antibody screening.
B cell maturation process, comparable to human adaptive immunity
Antibody candidates with the highest success rate in clinical trials *
Development Process of Fully Human Antibodies utilizing SynThese™ Transgenic Mouse
-
01
-
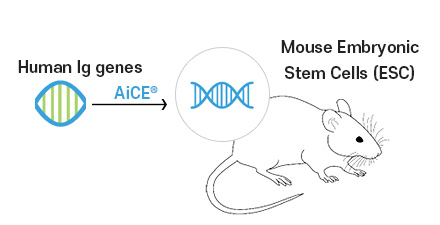
Chromosome-level
human Ig gene transfer
into mouse ESCApplying AiCE®
Technology
-
02
-
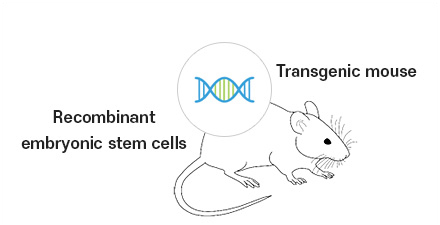
Development of SynThese™ transgenic mouse which has a full set of human Ig genes
-
03

-
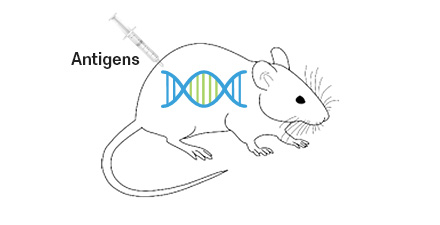
Immunization with
Target antigensHumoral immune
response
-
04
-
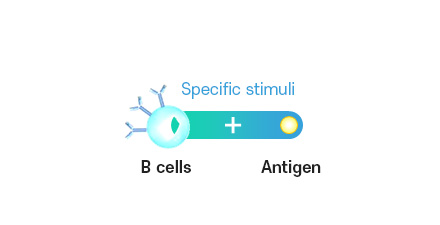
Antigen-directed B cell stimulation and proliferation
-
05
-
Selection of antigen-specific plasma B cells
-
06
-
Fully human mAb production /
Biophysical characterization
- Development of fully human (or humanized) monoclonal antibodies (mAb) using transgenic mouse platforms is recognized as a top-notch technology, displaying a technical superiority over the conventional Phage display antibody screening and the subsequent time-consuming IgG conversion process. A large number of human mAb antibody therapeutics have been developed from transgenic mouse platforms.
- SynThese™ platform, harnessing AiCE® technology, provides the most effective solution for developing fully human antibody therapeutics. This next-generation mouse platform enables us to generate fully potentiated mature antibody candidates against target antigens in a time-efficient manner.
- SynThese™ mouse platform establishes a fully potentiated mature mAb library against the target antigens by employing intact mouse B cell maturation process and natural selection of mAb candidates with improved antigen binding affinity and biophysical properties. Furthermore, SynThese™ mouse platform equipped with chromosome-level replacement of human Ig genes possesses the highest numbers of VDJ gene segments among transgenic mice platforms, increasing diversity of fully human mAb repertoire and thus, readily obtaining a variety of drug candidates for target indications.
Expected outcomes
-
01
Development of fully human antibodies
A higher success rate in clinical trials -
02
Antibody selection from the intact mouse B cell maturation
A highly stable and potent human antibody therapeutics -
03
Ready-to-test human antibody candidates
Shortening the time and efforts on R&D process
Beyond the Limitations : A Next-generation Transgenic Mouse Platform
Technological Evolution of Antibody Discovery Transgenic Mouse Platforms
 |
 |
 |
||||||
|---|---|---|---|---|---|---|---|---|
| 1st Generation | 2nd Generation | 2.5 Generation | 3rd Generation SynThese™ | |||||
| Core Technology | Origin of human Ig genes | BYC or YAC clone | BAC clone | Synthetic genes | Human chromosome | |||
| Genetic engineering method | Conventional Cloning | BAC Engineering | Gene synthesis | Targeted chromosome replacement | ||||
| Gene transfer strategy | Random Integration | Targeted Knock-In | Targeted Knock-In | Targeted Knock-In | ||||
| Functionality | Antibody maturation | Low | Middle-High | Middle-High | Middle-High | |||
| Antibody diversity | Low | Middle-High | Middle-High | High | ||||
| Platform flexibility | Middle | Middle-High | Middle-High | High | ||||
| Competitive Landscape | Developers | Medarex | Abgenix | Ligand | Kymab | Regeneron | Trianni | HuMab |
| Constant region | Human | Human | Rat | Mouse | Mouse | Mouse | Human | |
| Human VH diversity | 4 | 17 | 22 | 43 | 47 | 44 | 46 | |
| Human VLK diversity | 4 | 17 | 12 | 37 | 23 | 39 | 42 | |

High number of Antibody candidates
- Antibody diversity plus heterogeneity from AiCE®-based multiple antibody discovery platforms that encompass racial variations in human Ig genes
- High probability of retrieving rare mAb clones with a greater therapeutic potential
High success rate in Clinical trials
- Chromosome-level replacement with human immunoglobulin genes
- Fully human antibody derived from natural antibody maturation with the improved biophysical properties
Patent
-
Method for producing a transgenic non-human animal having a genome comprising a humanized immunoglobulin locus
- Technology
- SynThese™
- Filed
- KR, PCT

